GLOBAL CORAL REEF ALLIANCE WHITE PAPER
October 8, 2025
Electrifying coralline algae to regenerate white sand beaches and eroding islands against climate change
Thomas J. F. Goreau, PhD
President, Global Coral Reef Alliance
ABSTRACT
Increasing global sea level rise and storm strength, caused by global warming from fossil fuel Green House Gases (GHGs), are accelerating beach erosion worldwide, simultaneously with an increasing global crisis of suitable sand availability. Coralline Green and Red algae, especially Halimeda, are the most productive source of limestone in the ocean, and generate most tropical white beach sand, naturally replenishing sand inevitably lost to erosion. A single species, Halimeda opuntia, is the most important source of tropical white beach sand, having ideal genetic, physiological, and ecological adaptations for large-scale cultivation to regenerate beaches. With Biorock electric reef technology growing coralline algae directly in front of eroding beaches, while also protecting beach sand from wave erosion, natural white limestone sand beaches have been grown at record rates without any dredging, pumping, or dumping of sand. Examples from the Caribbean, Indian Ocean, Pacific, and South East Asia are shown. Actively growing beach sand where it is most needed makes sand supply no longer a zero-sum game, reliant on expensive sand shipments, stealing it from other beaches, rivers, or dredging the ocean and damaging coral reefs. Halimeda opuntia sand beaches can be grown around the tropics, and red coralline algae (Maerl) beaches in cold waters. A dozen sites around the world that would immediately benefit from Biorock sand farming technology are discussed. Matching sea level rise by actively growing new coralline algae sand supplies, while simultaneously protecting beaches from erosion using Biorock electric reef technology, creates a completely new strategy to save severely eroding beaches and low islands from sea level rise, with global applications for coastal climate change adaptation.
INTRODUCTION: THE GLOBAL BEACH SAND CRISIS
Beach erosion is accelerating worldwide (Luijendijk et al. 2018), a growing crisis caused by global sea level rise, increasing strength and frequency of extreme storms driven by global warming, increasing human-caused imbalance between sources and sinks of coastal sands caused by alterations to river and coastal hydrology, and destruction of ecosystems that protect beaches from waves and store Blue Carbon like coral reefs, oyster reefs, mangroves, sea grasses, and saltmarshes. It is forecast that half the world’s beaches will vanish this century (Vousdoukas et al., 2020). This projection assumes that both sea level rise and storm strength will not increase, when in fact both are accelerating faster than the highest climate model projections, making it a clear under-estimate! I propose here to use regenerative solutions powered by trickle charges of electricity, preferably solar, to grow coralline algae beach sand back and protect it from erosion, to keep pace with global sea level rise.
Sand beaches form on shores where sand supply exceeds erosion (Figure 1). Growing coral reefs lined most suitable tropical coasts, making white “coral” sand beaches the norm along tropical shores. Along rocky coasts beaches are confined to bays protected from open water wave erosion forces, which are compounded by reflection off smooth vertical surfaces, but dissipated by friction over rough horizontal surfaces. Wherever living, wave-breaking, reefs grow upwards faster than waves can break them physically apart, beaches form in their lee. Coral reefs are responsible for beaches along tropical shores, and cause the dramatic prevalence of white sand beaches along tropical coral-lined coasts, compared to temperate and cold coasts, or to tropical coasts without reefs. In cold waters, much smaller reefs made by oysters, mussels, and other colonial invertebrates can also protect sand beaches, unfortunately these ecosystem structural geo-engineers have been largely destroyed by overharvesting.
 Figure 1. Maldives beach sand is almost pure limestone. Approximately 30 cm across. Larger pieces are broken corals and shells. Hundreds of small rounded white flakes are Halimeda sand grains that have not been broken, and fine sand, silt, and mud is mostly derived from broken Halimeda. Sample collected by T. Goreau, Ihuru, North Male Atoll, 2001. Photograph 2024 by T. Goreau.
Figure 1. Maldives beach sand is almost pure limestone. Approximately 30 cm across. Larger pieces are broken corals and shells. Hundreds of small rounded white flakes are Halimeda sand grains that have not been broken, and fine sand, silt, and mud is mostly derived from broken Halimeda. Sample collected by T. Goreau, Ihuru, North Male Atoll, 2001. Photograph 2024 by T. Goreau.
Loss of coral reefs from global warming, pollution, new diseases, physical destruction by hurricanes, boring organisms, seawall-caused erosion, engineering construction and dredging, etc., have pushed coral reefs past their tipping points almost everywhere (Goreau & Hayes, 2021; 2023). Coral loss now causes increasing loss of tropical beaches worldwide after the solid wave-breaking reefs that used to protect beaches crumble internally from bio-erosion and collapse after storms, and new sand supplies from living reef organism skeletons vanish, changing sediment-rich coastlines with growing beaches into sediment-starved coasts, eroding as local physical wave forces increase from global warming and global sea level rise.
When Miami Beach decided they could make more money by doubling the width of the natural “coral sand” beach, the first sand they dumped on the beach in 1970 was quickly washed into the sea by storms, burying and killing the natural coral reef that had grown the original white sand beach. After the natural sand sources and shore protection from the reef were destroyed, Southeast Florida entered an endless cycle of frequent desperate dredge-dumping sand on the beach at the start of the tourist season, and watching the beach vanish in the first big storms (Wanless & Maier, 2007). Now they need to do it almost every year, like Cancun, Galveston, and many other places. Each cycle of sand dredging, dumping, and erosion by storms loses available sand that washes away into deep water, so now that there is no more dredgeable sand left in South Florida, they must import it at enormous cost (Shoemaker, 2025).
Sand shortages are making sand scarcer (Elko et al, 2021) and more costly (Rangel-Buitrago et all., 2023) worldwide. Most uses of sand are for the 80% of aggregate needed to mix with Portland Cement for concrete, or with petroleum tar for asphalt. Both have high carbon footprints, each ton of Portland Cement releases a ton of CO2 to the atmosphere, and almost all asphalt is refinery residue from petroleum distillation. Asphalt and Portland Cement are costly to transport long distances, and so is the 80% aggregate that must be added, causing further emissions, making concrete and asphalt production and transport major contributors to global CO2 emissions. Their total weight on earth now exceeds the biomass of living organisms (Figure 2., from Elhacham et al., 2020).
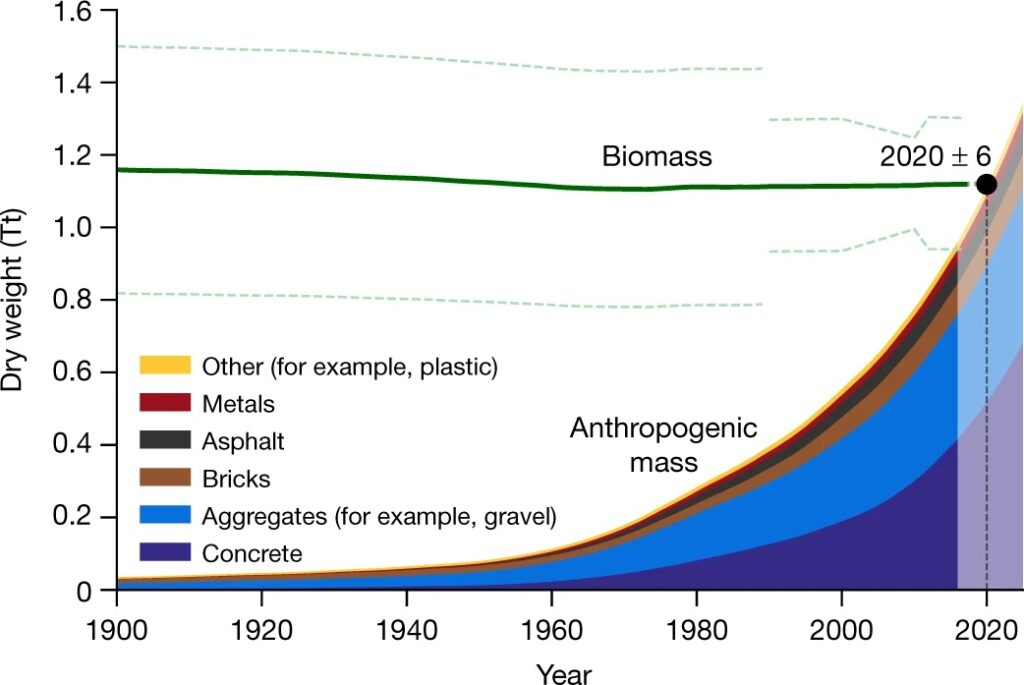 Figure 2. Global mass of construction aggregate (sand and gravel) and concrete now exceeds biomass on Earth (from Elhacham et al. 2020).
Figure 2. Global mass of construction aggregate (sand and gravel) and concrete now exceeds biomass on Earth (from Elhacham et al. 2020).
For sand blending applications the key factors are size, shape, and in particular, rough microscopic surface texture of grains so that Portland Cement or asphalt sticks to them. Smooth or rounded grains found on beaches or deserts are NOT suitable, preferred sources are actively moving river channel sands, which are intensively dredged, often by criminal syndicates, causing tremendous damage and pollution to downstream watersheds and coastlines, polluting them with mud and toxic chemicals, while causing erosion of coastal beaches by starving them of sediment supplies. Where river sand supplies have become inadequate for industrial and beach replenishment, entire beaches are being illegally dredged, stolen, and trucked off to eager buyers (Rangel-Buitrago et al., 2023).
Sand replenishment for eroded tourism beaches has become a global crisis as natural yellow sand supplies, derived from erosion of igneous rocks and transported by rivers, decreases due to trapping by reservoirs. Natural long-shore coastal wave and current-driven drift of sand is vanishing due to coastal yellow sand lost to dams on upstream rivers, and as longshore drift is intercepted by coastal groins and jetties, causing most previously growing or stable beaches to start disappearing.
Most non-tropical beach sand grains are composed of quartz (silica, SiO2) crystals eroded from mountains millions of years earlier. Here this is referred to as “Yellow sand”, mixed with detrital minerals, distinguishing yellow quartz sand from white tropical sand, which is made up entirely of limestone (calcium carbonate, CaCO3) remnants of the skeletons of living marine organisms, such as corals or shells. The famous tropical white sand beaches are universally called “coral sand”, but in fact almost every sand grain is the remnant of calcareous limestone skeletons of coralline Green or Red algae, and not of corals, snails, clams, or microscopic organisms such as foraminifera (Milliman, 1974), although those all can be locally important. While every grain of quartz and detrital mineral sand is ancient and has been transported from someplace else, every grain of limestone sand is the skeleton of a living organism, which are capable of sustainably producing sand unless they are killed by environmental changes, usually caused by humans.
CORALLINE ALGAE
Coralline Green and Red algae, first described by Aristotle, are ubiquitous in shallow, clear warm waters, and coralline Red algae are found in cold waters as well. Coralline Red algae grow in a wide range of marine habitats, from the intertidal down to the deepest algae growing in the ocean at the lowest possible light levels for photosynthesis (around 1% of surface levels, Littler & Littler, 2000; 2003). While around a dozen genera of Green and Red algae make limestone skeletons, the vast majority of limestone sand comes from species of the Green alga Halimeda, followed distantly by Green Udotea, Penicillus, Acetabularia, and other Green and Red genera. All Green calcareous algae grow vertically erect or hanging, segmented, branching, calcified, limestone plates that turn white, fall off, and become sand when the algae dies. Green coralline algae are never encrusting, but in contrast only a few Red coralline genera are branching, and most are flat, encrusting, and very slow growing, so they horizontally coat over and bind hard bottom rock substrate, cementing reef rubble together, but don’t produce beach sand.
Fast-growing branching Coralline Red Alga genera such as Corallina, Jania, Galaxaura, and Amphiroa are segmented and produce sand. The amount of sand they produce is generally far less than Halimeda, but unlike calcareous green algae that are confined to warm waters, coralline reds are also found in cold waters, producing limestone sand sediments such as maerl in waters too cold for the tropical coralline Green algae (Figures 3-6). Maerl and Halimeda have been harvested or dredged for use as agricultural lime in fields for thousands of years. Cold water maerl forming-algae grow slowly, only about one millimeter per year, yet they form oases of biodiversity in cold waters (Barbera et al, 2003; Peña & Barbara, 2008).
 Figure 3. Corallina ferreyrae growing in tide pool near Suevos, Galicia, Spain. About 50 cm across. Photograph by T. Goreau, 2025.
Figure 3. Corallina ferreyrae growing in tide pool near Suevos, Galicia, Spain. About 50 cm across. Photograph by T. Goreau, 2025.
 Figure 4. Bleached Corallina ferreyrae in tide pool. When the alga dies, the white limestone skeleton remains attached to the rock until waves break it off and convert it to sand. About 30 cm across. Photograph by T. Goreau, Suevos, 2025.
Figure 4. Bleached Corallina ferreyrae in tide pool. When the alga dies, the white limestone skeleton remains attached to the rock until waves break it off and convert it to sand. About 30 cm across. Photograph by T. Goreau, Suevos, 2025.
 Figure 5. Pterocladiella capillacea, alive. About 30 cm across. Photograph near Suevos, by T. Goreau, 2025.
Figure 5. Pterocladiella capillacea, alive. About 30 cm across. Photograph near Suevos, by T. Goreau, 2025.
 Figure 6. Lithophyllum byssoides encrusting tidal pools near Suevos, Galicia. About 30 cm across. Photograph by T. Goreau, 2025.
Figure 6. Lithophyllum byssoides encrusting tidal pools near Suevos, Galicia. About 30 cm across. Photograph by T. Goreau, 2025.
HALIMEDA IN THE GLOBAL CARBON CYCLE
Most limestone production in the ocean is by microscopic plankton, mainly coccolithophorids and foraminiferans, but only those falling onto shallow shelves are preserved in sediments, and most marine limestone production dissolves when it falls into the deep sea, where bottom waters are acidic from decomposition of planktonic organic carbon (Milliman, 1974; 1993; Milliman & Droxler, 1995; Mackenzie & Lerman, 2006). Coral reefs represented around half of the net limestone accumulation in the ocean back when coral reefs were at their prime, even though they occupied less than 0.1% of the ocean bottom (Milliman, 1974). Those estimates predate mass mortality of corals from global warming (Goreau and Hayes, 2021). While coral limestone production has since collapsed, coralline algae survive higher temperatures than corals, yet they also are close to their upper limits (Vazquez-Elizondo & Enriquez, 2016). Coralline algae are killed by ocean acidification, especially the encrusting and finely branched Red algae (McKoy & Kamenos, 2014), and by diseases (Litler & Littler, 1995; Goreau et al., 1998; Cervino et al, 2005).
Coralline algae are dominant in low nutrient environments such as Central Pacific atolls, but when coastal nutrients increase from land-based sources of pollution, the first victims are the coralline algae, which are overgrown and killed by fleshy or slimy weedy algae, which don’t produce limestone sand or bedrock. There has been a collapse in new sand production in coastal waters polluted by land-based sources of nutrients from sewage and fertilizer. Restoring sand production in these areas requires that nutrients be removed by proper sewage treatment before discharge to the ocean (Goreau, 2003; DeGeorges et al. 2010).
Weedy algae smothering coral reefs in Kaneohe Bay, Oahu, Honolulu died back after installation of a long sewage pipe (designed only to dilute point sewage sources in coastal waters by moving them further offshore without treating them), but that limited progress was later reversed when non-point nutrient sources from suburban street runoff, lawn fertilizers, and other sources not entering the sewage system overwhelmed the reef again and the weeds returned (Hunter & Evans, 1995). Weedy algae were permanently removed in Dragon Bay, Jamaica, when all sewage inputs were diverted (Goreau 2003). In Discovery Bay Jamaica, where the reefs and coralline algae had been killed by fleshy algae overgrowth (Goreau, 1992), weedy algae died back after sewage treatment was installed, and the old calcareous algae populations that had been present before pollution returned. Recovery was then locally reversed after captive dolphin pens were installed for cruise ship tourists, causing renewed smothering of seagrass by weedy algae, and closure of public beaches down-current from them due to skin, nose, throat, and eye infections, but the weeds died back and the ecosystem recovered again after Covid put an end to cruise ships and dolphin exploitation businesses went bankrupt (Goreau, 2020).
Halimeda, a tropical coralline Green alga genus with around 50 species, is the most productive source of limestone per unit area in the ocean because it grows exceptionally rapidly in clear tropical waters, and each carbon dioxide molecule fixed by photosynthesis produces a molecule of calcium carbonate, balancing alkalinity generation internally (Goreau, 1963; Borowitzka, 1984: Borowitzka and Larkum, 1977; 1987). The organic carbon soon decomposes back to CO2, but the limestone carbon will be sequestered forever, or until acid contacts it.
Halimeda skeletal sand grains are highly porous, and when broken release fine aragonite needles, microns in length, which can be resuspended and transported long distances by wave surge. These accumulate in the silt and clay size fractions in reef crevices and are washed by storm surge into deep water (Neumann & Land, 1975; Moore et al. 1976; Land, 1979; Liddell & Ohlhorst, 1981; 1987; 1988; 1992; Boss & Liddell, 1987). Although the individual skeletal units are sand sized, they are porous and friable, being composed of extremely small aragonite limestone crystals. Mechanical breakage produces large amounts of fine limestone silt and clay, whose extremely high surface area results in Halimeda skeletons being among the first to dissolve in acidified water, for example after storm-stirred Halimeda sediments pour over continental slopes into deep undersaturated waters (Johns & Moore, 1988). Coral reefs produce much more sand than they can hold, and Halimeda sand not washed onto beaches can travel far into deep water, making larger contributions to global ocean limestone formation and dissolution budgets than framework skeletal limestones like corals (Goreau et al. 1979).
Halimeda sand production ranges around 1-5 Kg/m2/yr of sand, and is thought to make up at least 10% of global limestone production (Hillis-Colinvaux, 1980; Drew, 1983; Drew & Abel 1988; Verbruggen et al., 2009; Granier, 2012; Castro-Sanguino et al. 2020), including most tropical beach and lagoon sands. Halimeda limestone production has probably been underestimated because most Halimeda sand production breaks down into microscopic crystals that are washed away into deep water by waves rather than accumulating on beaches or shallow sediments. Enormous Halimeda banks provide most of the deep sediments in large parts of the Caribbean Basin (Jensen et al. 1985; Freile et al. 1995), the South China Sea, Indonesia (Roberts et al. 1987), Pacific and Indian Ocean atoll lagoon sands, the Great Barrier Reef Lagoon, where huge recently discovered banks have been mapped (Reolid et al., 2024), and unmapped Halimeda banks are thought to be widespread in tropical shelf waters. The incredible limestone productivity of Halimeda makes up around 10% or more of global ocean limestone sources, produced in an area of less than 0.1% of the ocean, or a limestone productivity per unit area more than 100 times greater than the ocean as a whole!
HALIMEDA OPUNTIA: SUPERSTAR SAND PRODUCER
Halimeda opuntia, the dominant shallow water species (Goreau, 1959; 1963; Goreau & Goreau, 1973) forms dense mats near shore that were first collected, along with branching Red coralline algae, in Kingston Harbour, Jamaica by Hans Sloane in 1687-1689 (Figures 7 & 8, from Sloane, 1707; Figure 9 from Ellis & Solander, 1786).
 Figure 7. Halimeda opuntia, Kingston Harbour, Jamaica, collected 1687-1689, from Hans Sloane, Natural History of Jamaica, 1707.
Figure 7. Halimeda opuntia, Kingston Harbour, Jamaica, collected 1687-1689, from Hans Sloane, Natural History of Jamaica, 1707.
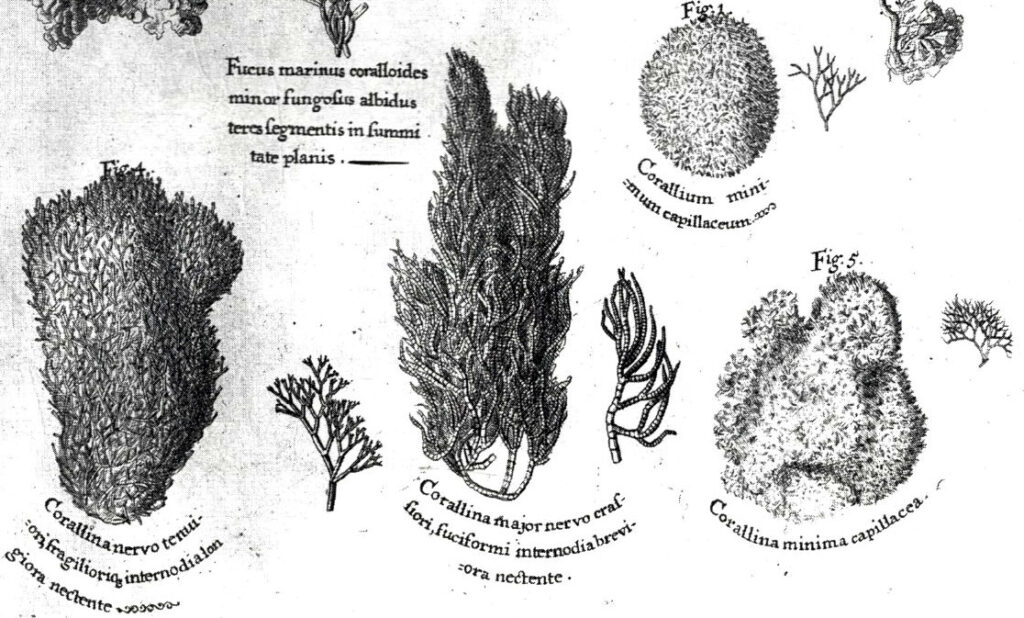 Figure 8. Branching Red coralline algae collected in Kingston Harbour, Jamaica 1687-1689. From Hans Sloane, 1707.
Figure 8. Branching Red coralline algae collected in Kingston Harbour, Jamaica 1687-1689. From Hans Sloane, 1707.
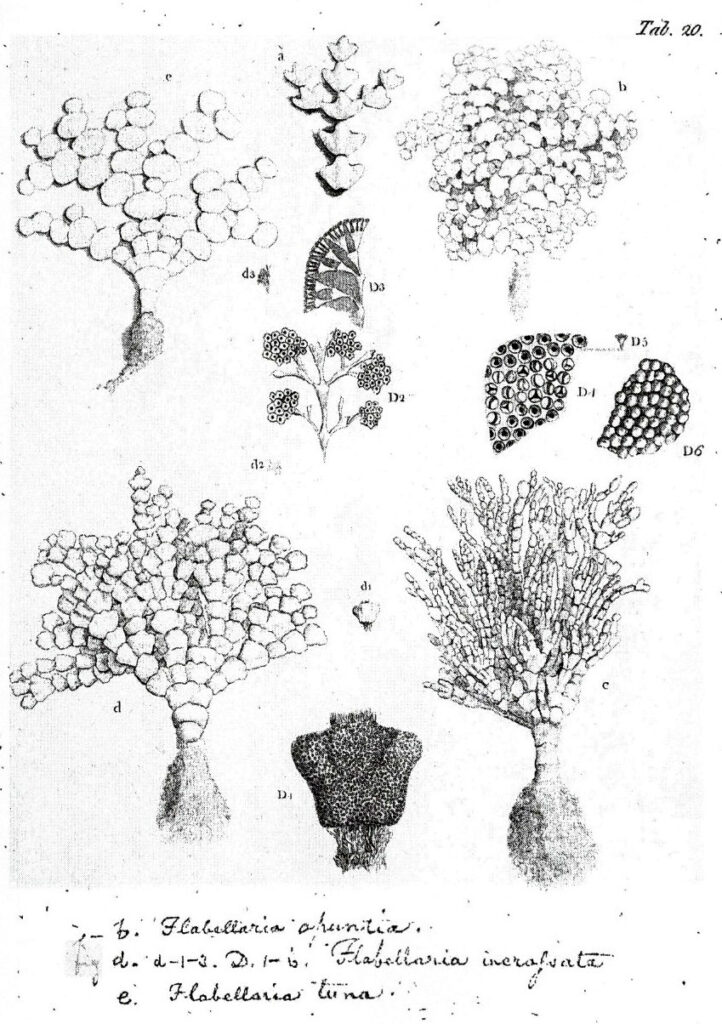 Figure 9. Halimeda opuntia (upper right and lower left), Halimeda incrassata (lower right), and Halimeda tuna (upper left), Ellis and Solander (1786).
Figure 9. Halimeda opuntia (upper right and lower left), Halimeda incrassata (lower right), and Halimeda tuna (upper left), Ellis and Solander (1786).
Because of its limestone skeleton, Halimeda is palatable to only a few parrotfish species with suitable crushing teeth (Muñoz & Motta, 2000), and is primarily found in shallow water with high wave action that deters herbivores, or in deep waters with less fish (Goreau & Goreau, 1973). In herbivore-dominated coral reefs Halimeda is confined to growing in crevices where fish and sea urchins can’t reach and eat them, but where grazing is reduced, often due to overfishing or wave action, Halimeda forms enormous banks, kilometers across (Reolid et al., 2024).
Halimeda beach sand supplies are very sensitive to water quality. Coralline algae dominate only in oligotrophic reefs with very low nutrients. As nutrients increase from very low levels, the biomass of Halimeda opuntia can greatly increase in seagrasses, lagoons, and nearshore areas to the point that small corals can be smothered (Goreau, 1992). Halimeda and coralline algae in general cannot stand fresh water or eutrophication: as nutrients increase, soft, fleshy, or slimy, non-calcareous algae weeds proliferate, overgrowing, smothering, and killing sand-producing coralline algae as well as corals (Goreau, 1992), terminating sand supplies to the beach and greatly increasing beach erosion. Areas with high sewage inputs are first to lose their Halimeda beach sand supply to soft algae that produce no sediments and smother and kill coralline algae that do.
Around 50 species of Halimeda have been described, with many of the most abundant species pan-tropical in distribution (Kooistra et al., 2002; Verbruggen & Kooistra, 2004; Kooistra & Verbruggen, 2005; Verbruggen et al. 2005; Verbruggen et al., 2009; Zhang et al., 2024). Ecological studies shows that Halimeda species are strongly zoned by depth (Goreau & Goreau, 1973), with strong effects on the fate of the sand they produce.
The most abundant and prolific species, Halimeda opuntia, grows in very shallow seagrass beds in front of beaches, and provides the vast majority of beach sand. Sand grains from other Halimeda species such as H. tuna, H. monile, H. incrassata, H. discoidea, H. gracilis, H. lacrimosa, H. simulans, H. trilobata, etc. growing in the wave breaking reef may be washed onto the beach but are more likely to be washed into the back reef lagoon or out into deep water through sand channels pouring down the deep slopes. The bottoms of these channels largely consist of sand-sized Halimeda skeletal plates, coral fragments from storm breakage, and silt sized grains produced by the boring sponge Cliona (Goreau & Goreau, 1973; Goreau et al., 1979). On the fore-reef and deep-reef slopes, sand from the dominant deep reef Halimeda species like H. goreaui, H. copiosa, and H. cryptica, falls down the fore reef slope creating enormous sediment fans reaching abyssal depths, whose mass and volume are poorly known, but which form a very significant part of the ocean’s calcium carbonate budget, especially on tropical continental shelves and slopes (Milliman & Droxler, 1995).
The extraordinary growth of Halimeda was shown in the first marine autoradiographs, taken by the author’s grandfather at Bikini Atoll in 1946 (Figure 10, from LIFE, 1946). He placed the alga on a photographic plate and it photographed itself by radiation from radioactive Strontium-90 from nuclear bomb blasts incorporated into the skeleton.
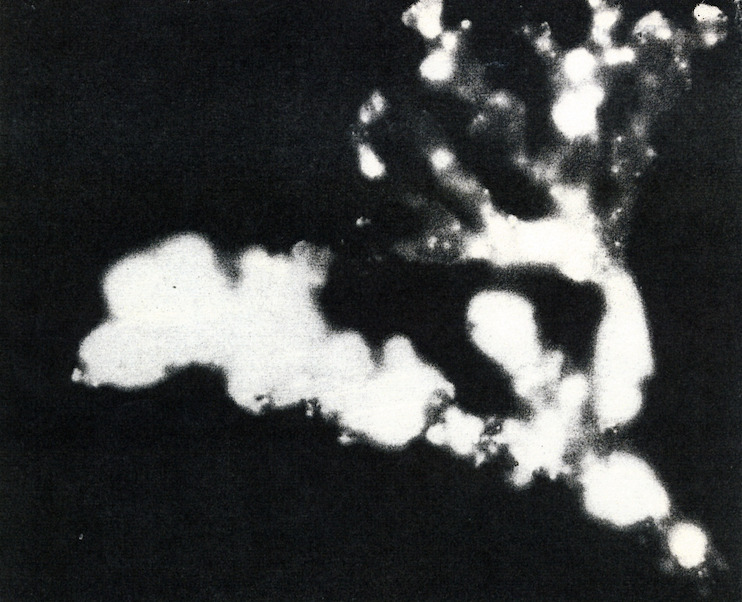 Figure 10. Halimeda photographed by its own radioactivity absorbed from Bikini nuclear bombs. The fastest growing parts are whitest, dark segments have stopped growing. Photograph 1946, Fritz Goreau (F. W. Goro), LIFE Magazine.
Figure 10. Halimeda photographed by its own radioactivity absorbed from Bikini nuclear bombs. The fastest growing parts are whitest, dark segments have stopped growing. Photograph 1946, Fritz Goreau (F. W. Goro), LIFE Magazine.
Halimeda opuntia is the unexcelled superstar limestone producer. Plants have been seen to grow in 24 hours (Goreau, 1963). Extraordinary Halimeda productivity is due to many evolutionary genetic adaptations for rapid coupling of photosynthesis, organic carbon production, and carbonate limestone growth in nutrient-poor environments. The exceptional limestone productivity of Halimeda is due to the ability to calcify most rapidly (Bohm, 1973), and while all other skeleton calcification is intracellular, Halimeda skeletons uniquely precipitate limestone skeletons in extracellular spaces that that have been closed off from seawater by algae filaments (Borowitzka and Larkum, 1977). This allows them to grow skeletons in acidic waters (Vogel, et al, 2015), which kill crustose coralline Red algae. Halimeda undergoes genome duplication without forming cell walls between daughter cells, so the entire plant is multi-nucleate and coenocytic, and behaves like a single huge cell with many nuclei, each expressing selected portions of the genome (Zhang et al., 2024).
Halimeda opuntia evolution shows high expression and integration of enzymes that link calcification to photosynthesis (Zhang et al., 2024). It forms dense mats up to 20-50 cm high on seagrass and sand bottoms, where it grows attached to broken coral or shell fragments. One reason for its abundance is that while most Halimeda species branch new segments along a line, or a single plane, and grow either vertically upward or hang down, and are attached at a single point, Halimeda opuntia has regulatory gene mutations that allows it to branch in three dimensions. This morphology allows it to sprawl and proliferate as dense mats over shallow back reef and sandy environments in front of the beaches it feeds with sand, producing up to around 5 kilograms of sand per square meter per year, and as shown below, likely even more with Biorock electric reef stimulation. Halimeda opuntia attaches to bottom hard surfaces at many places allowing it to spread rapidly and regenerate from small fragments (Verbruggen et al., 2009). While other species form small individual plants growing from single attachment points, Halimeda opuntia forms huge beds where individual plants cannot be distinguished. Halimeda opuntia is worldwide in distribution, but could occupy larger suitable habitat areas than it actually does, apparently being recruitment-limited (Verbruggen et al., 2009).
The evolution of Halimeda opuntia is distinguished by multiple genome reorganizations followed by a massive polyploid genome multiplication event 3.4 million years ago (Zhang et al., 2024), near the start of the Ice Ages, so subsequent Pleistocene global sea level changes led to widespread pan-tropical proliferation (Zhang et al., 2024). Wide Halimeda white sand beaches along tropical shores and sand bar islands on reef crests probably did not exist before the Pleistocene Ice Ages. Halimeda opuntia dominates shallow waters with high light levels due to its unique three-dimensional branching pattern. Similar genetic histories for the dominant Caribbean deep water, low-light species like Halimeda copiosa (Figure 11) and Halimeda goreaui (Figure 12) have not yet been carried out.
 Figure 11. Halimeda copiosa growing on the deep fore reef wall in Jamaica. The prolific production falls into deep water. Photograph by Thomas F. Goreau, 1960s.
Figure 11. Halimeda copiosa growing on the deep fore reef wall in Jamaica. The prolific production falls into deep water. Photograph by Thomas F. Goreau, 1960s.
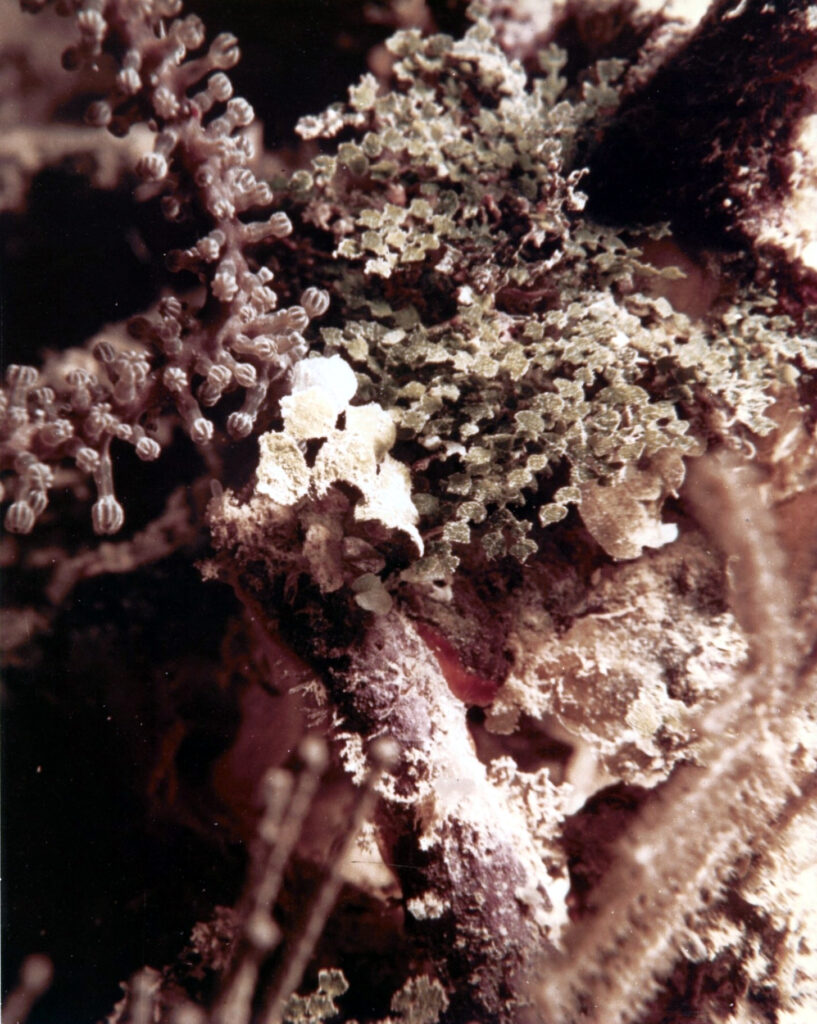 Figure 12. This photo taken in Jamaica by Thomas F. Goreau in the 1960s shows the two dominant deep fore reef wall sand producers. Halimeda copiosa is the larger species in the centre, the smaller more abundant species may be the first record of what was later classified as Halimeda goreaui (Taylor, 1962)
Figure 12. This photo taken in Jamaica by Thomas F. Goreau in the 1960s shows the two dominant deep fore reef wall sand producers. Halimeda copiosa is the larger species in the centre, the smaller more abundant species may be the first record of what was later classified as Halimeda goreaui (Taylor, 1962)
RESULTS: GROWING CORALLINE ALGAE WITH ELECTRICITY
Biorock electric reef technology uses safe, extremely low voltage (SELV) direct electrical currents to grow limestone reefs of any size or shape (Goreau & Trench, 2012) that greatly stimulate natural biological processes such as marine organism settlement, growth, survival, healing, reproduction, and resistance to extreme stress of all marine organisms looked at (Goreau, 2022; 2023). The limestone minerals produced are up to three times stronger than concrete, cheaper to produce with inexpensive solar power, and can be grown where needed so they don’t need to be transported long distances. Not only are transport costs eliminated, instead of producing a ton of CO2 for each ton of Portland Cement, Biorock limestone is CO2 neutral or negative, and therefore helps reduce global warming (Hilbertz, 1992; Goreau, 2012). Biorock electric reefs grown in front of severely eroded beaches reduce wave energy at the shore so beach sand grows back by itself without any dredging, pumping, or dumping of sand imported from external sources (Goreau & Prong, 2017).
The first Biorock reef constructed in the 1980s at the Discovery Bay Marine Laboratory in Jamaica was in a reef that had been almost completely killed by weedy algae overgrowth, over-fertilized by untreated sewage effluents (Goreau 1992). Long, slimy green algae mats had killed most coral and Halimeda at the site, and we expected weedy algae to overgrow the new Biorock reef as well. To our astonishment, coral fragments transplanted to Biorock reefs grew at record rates, at sites where corals were dying from poor water quality, and Biorock reefs were largely free of weedy algae smothering nearby reefs. Instead of weedy algae, prolific, rapidly-growing clumps of the branching Red sand-producing coralline alga, Jania rubens, overgrew the tops and sides of the reef, with Halimeda growing around the base (Figures 13-15), and sand began to accumulate again on the eroded bottom (Goreau & Hilbertz, 2012). The eroded limestone bedrock rock that the Biorock reef was built on had previously been a Halimeda sand bed overgrown by seagrass, but a couple meters of sand were washed away in Hurricane Allen (1980), and never came back. Besides sand, Jania rubens and Halimeda yield economically-useful organic chemicals and hormones, some that have anti-cancer activities and others claimed to reduce skin aging by ultraviolet light (Chenniappan et all., 2021; Naiel et al, 2025).
 Figure 13. Biorock reef, with prolific growth of Porites porites corals and large masses of coralline red algae on top. Discovery Bay Marine Laboratory, Jamaica, about two years old. Photograph 1991 by Dr. Peter D. E. Goreau.
Figure 13. Biorock reef, with prolific growth of Porites porites corals and large masses of coralline red algae on top. Discovery Bay Marine Laboratory, Jamaica, about two years old. Photograph 1991 by Dr. Peter D. E. Goreau.
 Figure 14. Coralline red algae on top of Biorock structures, Discovery Bay Marine Laboratory, Jamaica. Image about 10 cm across. Notice similarity to Sloan drawing (Figure 8) Photograph 1991 by Dr. Peter D. E. Goreau.
Figure 14. Coralline red algae on top of Biorock structures, Discovery Bay Marine Laboratory, Jamaica. Image about 10 cm across. Notice similarity to Sloan drawing (Figure 8) Photograph 1991 by Dr. Peter D. E. Goreau.
 Figure 15. Masses of coralline red algae growth between corals on the vertical sides resulted from spontaneous settlement and growth. Below it is a zone of Halimeda. Discovery Bay Marine Laboratory, Jamaica, photograph 1991 by Dr. Peter D. E. Goreau. Sand is accumulating on the hard rock bottom. The limestone on which the Biorock reef has cemented itself so strongly as to be unaffected by Category 5 Hurricane Gilbert (1988), had been buried underneath several meters of Halimeda sand and sea grass beds that were completely washed away during Hurricane Allen in 1980 (Goreau, 1992).
Figure 15. Masses of coralline red algae growth between corals on the vertical sides resulted from spontaneous settlement and growth. Below it is a zone of Halimeda. Discovery Bay Marine Laboratory, Jamaica, photograph 1991 by Dr. Peter D. E. Goreau. Sand is accumulating on the hard rock bottom. The limestone on which the Biorock reef has cemented itself so strongly as to be unaffected by Category 5 Hurricane Gilbert (1988), had been buried underneath several meters of Halimeda sand and sea grass beds that were completely washed away during Hurricane Allen in 1980 (Goreau, 1992).
In Pemuteran, Bali, Indonesia, around 150 Biorock reefs have been grown since 2000 on deep sand in front of a coral reef that had been killed by bleaching in 1998. After the corals died, the sand beach behind the dead reef began to erode faster, and trees lining the shore were collapsing into the sea. To stop trees and beach chairs falling into the water, the hotel built a small sea wall, which accelerated erosion as waves reflected off the vertical concrete wall and washed away the sand, first in front, then underneath. The beach had originally been black volcanic sand that burned bare feet if you carried heavy scuba tanks on your back. Coralline algae spontaneously began to grow on, under, and around the offshore Biorock reefs and white sand began to accumulate on the bottom over dark mud from volcanic soils washed down the mountainside. Halimeda rapidly proliferated on and around Biorock reefs (Figures 16-19). After 10 years of Biorock reef growth we realized that the beach had grown back all by itself, and changed color from black to a much lighter color due to the amount of white limestone sand washing in from the Biorock reefs, and protected by them (Figures 20-22).
 Figure 16. Halimeda opuntia growing on Biorock reef, Pemuteran, Bali. Green ovals outline Halimeda mats.
Figure 16. Halimeda opuntia growing on Biorock reef, Pemuteran, Bali. Green ovals outline Halimeda mats.
 Figure 17. Halimeda opuntia growing on Biorock reef (green oval), Pemuteran, Bali.
Figure 17. Halimeda opuntia growing on Biorock reef (green oval), Pemuteran, Bali.
 Figure 18. Halimeda opuntia (green oval) growing behind giant clam on Biorock reef, Pemuteran, Bali,.
Figure 18. Halimeda opuntia (green oval) growing behind giant clam on Biorock reef, Pemuteran, Bali,.
 Figure 19. Branching pink coralline algae (red ovals) growing nearby Halimeda on Biorock reef, Pemuteran, Bali.
Figure 19. Branching pink coralline algae (red ovals) growing nearby Halimeda on Biorock reef, Pemuteran, Bali.
 Figure 20. Taman Sari beach at low tide. At the start of the Biorock projects in 2000, the trees were collapsing into the sea, the entire beach was underwater at high tide, and beach chairs could not be left out without floating away. Photograph 2019 by T. Goreau.
Figure 20. Taman Sari beach at low tide. At the start of the Biorock projects in 2000, the trees were collapsing into the sea, the entire beach was underwater at high tide, and beach chairs could not be left out without floating away. Photograph 2019 by T. Goreau.
 Figure 21. The beach at Taman Sari, Pemuteran, Bali, at low tide. The change in sand colour marks the previous high tide, wet sand is dark and sand above the high tide mark is white. Before the Biorock project began in 2000 the beach was made up of black volcanic sand eroded from the black basalt lava in the background. The Biorock sand production offshore changed the colour of the beach from black to white, making it much easier to walk across barefoot with a SCUBA tank on your back. Photograph 2019 by T. Goreau.
Figure 21. The beach at Taman Sari, Pemuteran, Bali, at low tide. The change in sand colour marks the previous high tide, wet sand is dark and sand above the high tide mark is white. Before the Biorock project began in 2000 the beach was made up of black volcanic sand eroded from the black basalt lava in the background. The Biorock sand production offshore changed the colour of the beach from black to white, making it much easier to walk across barefoot with a SCUBA tank on your back. Photograph 2019 by T. Goreau.
 Figure 22. Black basalt lava bedrock on the beach at Taman Sari, Pemuteran, Bali. The white sand burying it is mostly made up of Halimeda sand produced by more than 100 Biorock reefs in front of the beach. Photograph 2019 by T. Goreau.
Figure 22. Black basalt lava bedrock on the beach at Taman Sari, Pemuteran, Bali. The white sand burying it is mostly made up of Halimeda sand produced by more than 100 Biorock reefs in front of the beach. Photograph 2019 by T. Goreau.
Biorock reefs grew back a severely-eroded beach at Pulau Gangga, Sulawesi, Indonesia at record rates naturally in less than a year (Goreau & Prong, 2017). The beach grew in height about 1.5 metres, increased in width about 20 metres, and in length about 400 metres (Figures 23-25), with large amounts of new Red coralline algae (Figure 26). This Sulawesi beach grew about as much over two years as the Bali beach did over 20 years. The difference reflects the greater wave energy at the Bali site, which has no reef crest or back reef flat in front of the beach, while the Pulau Gangga site has a coral reef crest emergent at low tide and a large shallow sand flat between the crest and the beach that was nearly dry at low tide.
 Figure 23. The Biorock-grown beach at Pulau Gangga, Minahasa, Sulawesi was grown by Biorock reefs (dark structures at right, submerged at high tide). When the project began in 2015 the beach nearly vanished at high tide, there was a 1.5 meter high erosional cliff, and the wooden buildings were collapsing into the sea. Low tide photograph 2018 by T. Goreau.
Figure 23. The Biorock-grown beach at Pulau Gangga, Minahasa, Sulawesi was grown by Biorock reefs (dark structures at right, submerged at high tide). When the project began in 2015 the beach nearly vanished at high tide, there was a 1.5 meter high erosional cliff, and the wooden buildings were collapsing into the sea. Low tide photograph 2018 by T. Goreau.
 Figure 24. The tree shown here collapsed horizontally into the sea over an erosional cliff in 2014, but remained rooted, and one side branch grew back vertically into a new tree after the new sand buried it. The two concrete pedestals were the bases of the cabana at the left, which had to be moved inland before it fell into the sea. Photograph 2018 by T. Goreau.
Figure 24. The tree shown here collapsed horizontally into the sea over an erosional cliff in 2014, but remained rooted, and one side branch grew back vertically into a new tree after the new sand buried it. The two concrete pedestals were the bases of the cabana at the left, which had to be moved inland before it fell into the sea. Photograph 2018 by T. Goreau.
 Figure 25. Measuring the height and width of the new beach profile at Pulau Gangga. The convex growing beach profile had been concave and eroding at the start of the project. Photograph 2018 by T. Goreau.
Figure 25. Measuring the height and width of the new beach profile at Pulau Gangga. The convex growing beach profile had been concave and eroding at the start of the project. Photograph 2018 by T. Goreau.
 Figure 26. Dead algae on the Pulau Gangga beach, the day after a severe storm covered the sand with dead algae and seagrass (long dark leaves). Most is coralline branching red algae. One clump at centre (red arrow, red oval) has the original red colours, but most are pink and bleaching (pink ovals) in the sun as they dry out and pigments break down, while the clump at lower right (white oval, white arrow) has already lost all its colour and turned bright white. In a few sunny days they will all be white and become sand. About 30 cm across. Photograph 2019 by T. Goreau.
Figure 26. Dead algae on the Pulau Gangga beach, the day after a severe storm covered the sand with dead algae and seagrass (long dark leaves). Most is coralline branching red algae. One clump at centre (red arrow, red oval) has the original red colours, but most are pink and bleaching (pink ovals) in the sun as they dry out and pigments break down, while the clump at lower right (white oval, white arrow) has already lost all its colour and turned bright white. In a few sunny days they will all be white and become sand. About 30 cm across. Photograph 2019 by T. Goreau.
In Gujarat, India, Biorock reefs were built by the Zoological Survey of India with the financial assistance from the Forest Department of Gujarat. One at Mithapur and another a few kilometres away at Arambhada were installed by students trained by Dr. Chowdula Satyanarayana, the top Indian coral expert, and the author. Both Biorock reef projects were powered by solar panel buoys. These are the first successful Biorock reefs installed in Indian waters and still going strong, despite suffering extreme temperatures, mud, wave energy, and tidal currents, as well as pollution from India’s major industrial chemical plants and oil tankers from the Persian Gulf headed for the world’s largest oil refineries in Jamnagar. The corals did very well, surviving severe bleaching, and Halimeda and crustose coralline algae began to grow above and underneath the structures, creating white sand patches under and around Biorock reefs (Figures 27-28).
 Figure 27. Halimeda tuna at right, near base of Biorock structures. The light green limestone plates are about a centimeter across. Of all the algal biomass in this image, only the Halimeda will contribute to the sediments, all the fleshy green and red algae biomass will decompose. Large plates are about a centimeter across. Halimeda appears to dominate the sediments. Photo 2023 by Dr. Chowdula Satyanarayana.
Figure 27. Halimeda tuna at right, near base of Biorock structures. The light green limestone plates are about a centimeter across. Of all the algal biomass in this image, only the Halimeda will contribute to the sediments, all the fleshy green and red algae biomass will decompose. Large plates are about a centimeter across. Halimeda appears to dominate the sediments. Photo 2023 by Dr. Chowdula Satyanarayana.
 Figure 28. Halimeda tuna growing near Biorock structures are dark green when young, get paler as they age due to increased limestone growth, and then turn white as the organic matter decomposes, leaving dead white limestone plates attached to the alga until wave surge breaks them off and they pile up as sediment on the bottom. The small size of fragmented Halimeda grains is shown by the fine white sediment dusting the living algae. A long ropy egg case of the large shell-less snail, the Sea Hare Aplysia sp. has been laid coiled up at the base of the plant. Photo 2025 by Dr. Chowdula Satyanarayana.
Figure 28. Halimeda tuna growing near Biorock structures are dark green when young, get paler as they age due to increased limestone growth, and then turn white as the organic matter decomposes, leaving dead white limestone plates attached to the alga until wave surge breaks them off and they pile up as sediment on the bottom. The small size of fragmented Halimeda grains is shown by the fine white sediment dusting the living algae. A long ropy egg case of the large shell-less snail, the Sea Hare Aplysia sp. has been laid coiled up at the base of the plant. Photo 2025 by Dr. Chowdula Satyanarayana.
In Velaa Resort, located in the world’s largest atoll, in northern Maldives, Biorock reefs were spontaneously settled on by coralline algae, and white sand began accumulating under them in a year (Figure 29).
 Figure 29. Halimeda opuntia, showing a colony spreading across the bottom at Velaa, Malidves, approximately 20 cm across, growing below a Biorock reef project. Photo 2025 by Agne Griciute. The three-dimensional branching is clearly seen.
Figure 29. Halimeda opuntia, showing a colony spreading across the bottom at Velaa, Malidves, approximately 20 cm across, growing below a Biorock reef project. Photo 2025 by Agne Griciute. The three-dimensional branching is clearly seen.
Biorock technology allows, for the first time, new natural white limestone “coral” sand beaches to grown through a two-pronged strategy: growing Halimeda sand “factories” on sea grasses where wave action will transport it onto the beach, and protecting the beach from wave erosion with a growing wave-absorbent Biorock reef. Biorock turns limestone sand-starved habitats into sand-rich habitats to protect beaches and islands from coral mortality from high temperatures while growing shorelines upward to match sea level rise. While no work has yet been done with cold-water maerl, it is likely that its growth would also be stimulated, like the tropical branching red calcareous species.
PILOT PROJECTS FOR BIOROCK SAND PRODUCTION
The Global Coral Reef Alliance, the non-profit research and management organization that invented and developed Biorock electric reef technology, is planning pilot Biorock sand factory projects with local communities with critical beach erosion problems around the world (Figure 30). GCRA seeks funding for cutting-edge research on restoration and sustainable management of coral reefs in Small Island Developing States and islands.
 Figure 30. A design for a Biorock beach sand factory. Figure by T. P. Sarkisian. The Biorock reef both produces beach sand and protects it from erosion.
Figure 30. A design for a Biorock beach sand factory. Figure by T. P. Sarkisian. The Biorock reef both produces beach sand and protects it from erosion.
A dozen selected proposed projects and their potential benefits are discussed briefly below (Figure 31). The author has personally studied all but one of these sites for decades, and an outline of environmental and human histories of almost all locations can be found in Goreau (2024). These are only a few of the locations where we have worked longest, there are many more that could be included, coastal erosion is everywhere, and hopes of a future Blue Economy will be an illusion unless it is reversed. Only a handful of these locations have potential to raise private funds for beach regeneration, as there is now NO support for long term shore protection from any Government or funding agency. Hopefully islands that are lucky enough to have access to private financial resources to grow back their own beach, like Grand Cayman, Saint Barthelemy, and Velaa Private Island will invest in their own beaches and help those who lack the funds and are being abandoned to drown.
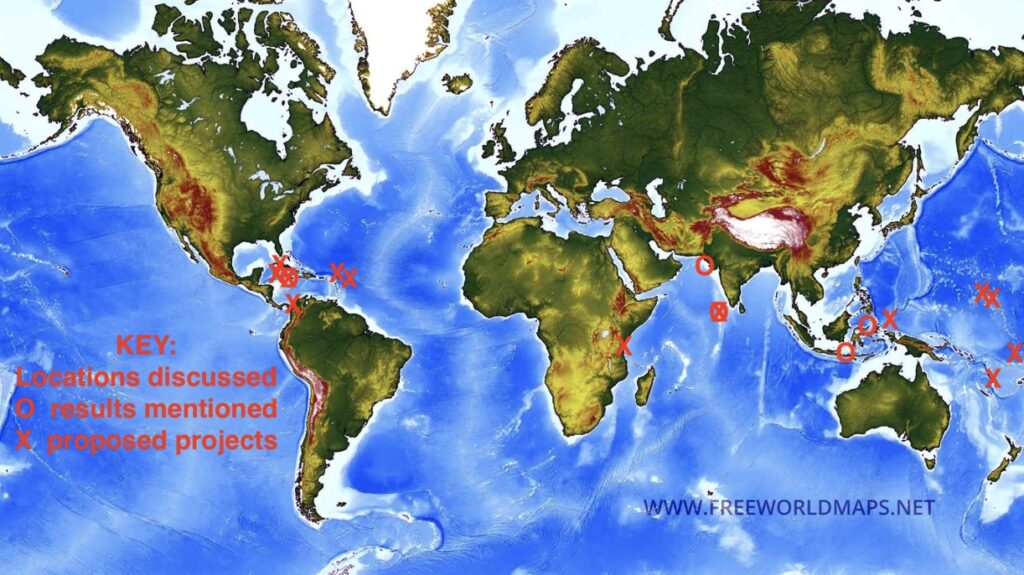 Figure 31. Locations of sites discussed. Circles are where previous results are discussed, crosses are proposed pilot beach regeneration projects
Figure 31. Locations of sites discussed. Circles are where previous results are discussed, crosses are proposed pilot beach regeneration projects
- Ejit and Kili, Marshall Islands
In 1946 the inhabitants of Bikini Atoll, master sailors who crossed the Pacific to Bikini some 3,000 years before, were suddenly expelled by the US Military, who blew up nuclear bombs and made their atoll uninhabitable. The Bikinians were dumped on a remote uninhabitable island with no lagoon, fish, or fresh water, where many starved, and then were irradiated by nuclear fallout from the US bomb tests from their own, now forbidden, island upwind (Niedenthal, 2013). Many died or suffered excruciating radiation burns and cancers. The survivors were evacuated again, and spent nearly a year in tents on the side of the runway of a US military bomber base on another island, and then finally dumped on Kili, a remote island uninhabitable because it had no lagoon or fish. Kili has no beach on which boats can safely land since there is no lagoon, it is too rough to fish more than a few days a year, and most coconuts and fruit trees have been killed by sea water flooding, which has contaminated the wells with salt water, taking place around the two largest tides of the year, and getting progressively worse as sea level rises. The author dived around Kili, and found that almost all corals had died in the 2016 bleaching. Our goal there is to work with the Kili people to grow back the beach at the site of worst erosion and seawater flooding.
Because Kili is so isolated, they were entirely dependent on an infrequent supply boat that frequently broke down, so people often went hungry since they couldn’t fish. Because of lack of employment, schools, and hospitals, many Bikinians migrated from Kili to the capital atoll Majuro, where they were permitted to live only on a separate very small island, Ejit. Ejit is isolated from the capital, so if boats are not available, Bikinians have to wait until low tide to wade knee-high or more through the rushing waters of three tidal channels to get to stores, hospitals, schools etc. Ejit floods with sea water increasingly, around twice a year. The corals are mostly dead, and Majuro atoll is severely overfished. Shore protection consists of dead corals and rubble packed into steel gabion baskets, which rust and fall apart in a year, or two if plastic coated (Goreau et al., 2007; Jormelu et al., 2010).
The author started a small solar-powered Biorock project at the Ejit elementary school, which floods twice a year, to protect it from sea level rise. The project used donated materials, and was so small as to only serve symbolic and heuristic educational lessons as to what Bikinians could do to protect themselves with minimal resources (Figures 32-33). Our goal is to expand the project to protect the school and all of Ejit from sea level rise, and ultimately other inhabited Marshallese islands.
 Figure 32. The Ejit primary school at low tide. During high King Tides seawater comes through the wall and floods school grounds and buildings. Photograph 2019 by T. Goreau.
Figure 32. The Ejit primary school at low tide. During high King Tides seawater comes through the wall and floods school grounds and buildings. Photograph 2019 by T. Goreau.
 Figure 33. The Ejit School from the reef flat at low tide. Sand is washed away because there is no intertidal reef to protect it from erosion. A Biorock reef built to protect the school (straight line in the water) is lying on the flat eroded limestone fossil reef in front of the school, powered by a solar panel on the roof. Photograph 2019 by T. Goreau.
Figure 33. The Ejit School from the reef flat at low tide. Sand is washed away because there is no intertidal reef to protect it from erosion. A Biorock reef built to protect the school (straight line in the water) is lying on the flat eroded limestone fossil reef in front of the school, powered by a solar panel on the roof. Photograph 2019 by T. Goreau.
- Funafuti, Tuvalu
Tuvalu is one of the world’s lowest countries, and around half the population is crowded into Funafuti Atoll, whose groundwater aquifer resources and taro food supply were destroyed in WWII by US military dredging to build a very briefly-used bomber airstrip. The only road connecting communities around the rim of the atoll is washing into the sea from both sides. The author filmed coral reefs all around Funafuti in 1997 helping train the first Tuvalu Marine Park Rangers, providing a baseline for changes in beaches and reefs, and discussed climate change adaptation at length with Toaripi Lauti, the first Prime Minister of Tuvalu, who fully grasped the issues.
Shore protection projects by international agencies are sea walls of imported concrete, rocks, and sandbags, which have all fallen down, many before they were completed. International funding agencies recently paid for a large dredge/dump landfill extension of the shore in downtown Vaiaku, the capital, but they did not have funds sufficient to protect the dumped sand landfill from wave erosion except by temporary use of imported plastic geotextile tubes pumped full of sand, which are notorious for collapsing in the first big storm, littering the beach with plastic!
On behalf of the Government of Tuvalu, the author prepared numerous proposals to use Biorock to protect shores from erosion, without any response from international funding agencies. The latest Tuvalu Biorock funding proposal is only for protecting floating solar panel arrays, not the people themselves. The Tuvalu Government has been waiting many years to hear from international funding agencies regarding funding, and while funding awaits, sea level rise continues! Our goal here is to regenerate coral reefs and sand supply in parts of urban Funafuti that were NOT included in the recent dredge dump landfill scheme.
- Hotsarihie, Palau
Hotsarihie means Reef of the Giant Clam in the language of the Hatahobei (Tobi) people who own it, but is usually shown on maps as Helen Reef, in the language of a much later European explorer. Hotsarihie is an isolated atoll, closer to New Guinea, Indonesia, and the Philippines than Palau itself. The Hatahobei and other remote southwest islanders lived by subsistence agriculture on remote islands without any lagoons for fishing, or even safe landing spots. Most SW islanders migrated to the main island of Palau for schools, hospitals, and employment, and live in a separate community speaking a different language. Hotsarihie was a 70 kilometer sail from Hatahobei, but is a huge atoll with a lagoon full of fish and was their only fishing ground. Hotsarihie has the highest biodiversity of corals, mollusks, fishes, and all marine life of any oceanic Pacific islands (Colin et al. 2008)), and was especially famous for the incredible abundance of huge giant clams. Hotsarihie is uninhabitable, the entire atoll is underwater at high tide except for a single tiny sandbar, covered with bird and turtle nests, which has no groundwater. The sandbar is migrating sideways about 15 metres a year across the reef flat, so rapidly that a coconut tree, the essential staple of atoll life, planted on the growing side of the island will collapse into the sea on the opposite eroding shore before it can bear coconuts. Hatahobei fishermen could only stay on Hotsarihie as long as their water and coconuts lasted before they had to go back home, and while they were away their island was massively plundered by industrial foreign fishing fleets that stole most of the large fish and giant clams. Almost all the sand on Hotsarihie is Halimeda, which grows prolifically all around and inside the atoll (Colin et al, 2008).
In 2000 the Governor of Hatahobei, Sabino Sackarias asked the author to help protect Hotsarihie from erosion so they could station a permanent ranger station to protect their atoll, and the rich tuna fisheries that surrounds it, from poachers. It took years to find funding to start, their only supply boat was broken and in dry dock for a couple of years for repair, so when we got to Hatahobei and Hostararie they had been completely isolated for several years, living only from fish and coconuts, and were desperate for rice and betel nuts! With the Hatahobei Marine Park Rangers, we built a 150 meters long solar panel powered Biorock reef. Unfortunately, we had inadequate funding, equipment, materials, electricity, people, food, and especially time. As soon as we finished building the solar powered reef we had to flee under emergency conditions due to a Super Typhoon, down to our last bag of rice (Goreau et al., 2004).
The Biorock shore protection reef we built was destroyed by huge logs during the typhoon but the solar panels were salvaged later by the Hatahobei Marine Park Rangers. Building a shore protection reef strong enough to withstand typhoons is not a problem with Biorock if suitable design and materials are available, which was unfortunately not the case the first time around! We have never found more funding to go back to finish the job with the Hatahobei people but we plan to if any funding can be found, as the problem is now more critical than ever (https://apnews.com/article/palau-helen-reef-island-hatohobei-coral-8d6bb9e4b4815d5e5b277d74f1c36277). It is urgent to save the Pacific’s unique and irreplaceable underwater paradise!
- Tanoliu, Efate, Vanuatu
Tanoliu is a traditional fishing village on the north side of Efate, the capital island of Vanuatu, one of the poorest, yet happiest, countries in the world. In 1942 the entire community were suddenly evicted from their lands by half a million US troops, who dredged up their coral reef to build a bomber air strip to attack the Solomon Islands, and then all left 6 months later to repeat the exercise in the next targeted island. Their coral reef and fisheries never recovered. To this day the shore is full of broken glass from beer bottles tossed into the sea and shot at from shore by American troops, but there is much more toxic military waste and weapons too.
The author taught a village Biorock coral reef and fisheries habitat restoration training workshop in Tanoliu funded by the United Nations Development Program (Goreau et al., 2016). This was very successful, and a dozen more villages requested training, but unfortunately funding could not be found to continue. Our goal is to finish growing back the reef and beach protecting the eroding shoreline in front of Tanoliu, which is threatening houses and roads, and use it as a training site for other villages. Most of the beach sand is Halimeda, which would rapidly proliferate if a Biorock reef protected it.
- Noonu, Maldives
The Maldives, unlike any of the Pacific atoll countries, is undergoing massive development of luxury resorts on eroding sand islands and artificial sand banks created by dredging, pumping, and dumping. Turbidity from dredging, pollution, and global warming have killed most of the corals (Goreau, 2024). Resort islands are surrounded by breakwaters of concrete, stone, sandbags, or dead corals in rusting gabions, to prevent the beach washing away. The availability of dredging equipment in the Maldives year-round to dredge sand for tourist islands has led to a side-industry dredging to expand inhabited islands as well as resorts, paid for by development bank funds rather than private investors. Because of large contracts to dredging companies, most inhabited (non-tourist) islands have suddenly found large amounts of sand dredged up from their lagoons and dumped on shore, often with little attention to local needs or long-term environmental impacts.
Here we propose to regenerate critical eroding beaches on several inhabited islands in Noonu Atoll, part of the world’s largest atoll, where shorelines and landfill are eroding. The author personally examined these islands and set up a pilot solar-powered Biorock project with community groups in front of an eroding dredged beach with failed geotextile tube shore protection projects, at a site with abundant Halimeda, Corallina, and Amphiroa in front (Figure 34). We propose to grow back sand beaches at eroding sites and propagate unique coral colonies that survived bleaching and are growing at record rates.
 Figure 34. Solar panel powering Biorock project. The dark patch in the water at past the end of the solar panel is a submerged geotextile tube from a previously failed “shore protection” project on which Halimeda and Jania are growing, with the intention of regrowing the now vanished beach to the left. Photograph by T. Goreau, 2025, Velidhoo Island, Noonu Atoll, Maldives.
Figure 34. Solar panel powering Biorock project. The dark patch in the water at past the end of the solar panel is a submerged geotextile tube from a previously failed “shore protection” project on which Halimeda and Jania are growing, with the intention of regrowing the now vanished beach to the left. Photograph by T. Goreau, 2025, Velidhoo Island, Noonu Atoll, Maldives.
- Sinda Island, Tanzania
Sinda Island is a small Marine Protected area near Dar es Salaam, the capital city of Tanzania, which recently suffered a devastating oil spill, whose damage is yet to be fully assessed although it killed mangroves, turtles, and completely destroyed the crops of local seaweed growers (Fiona Barreto, personal communication, 2025). Sinda Island is a Marine Protected Area popular with locals and tourists for swimming and snorkeling on the reef, on which the local economy depends. The reef has suffered badly from dynamite fishing and repeated bleaching events before the oil pollution. The nearest village on the mainland side was declared a forest reserve, but left unprotected, the mangroves were cut and sold for firewood after reef fisheries collapsed from dynamite fishing, so now the beach is eroding. Local women collect dead corals from the eroding beach and smash them into small gravel with hammers, which they sell for a dollar or two for a day of hard work.
We propose to work with a village community mangrove reforestation project to regrow the mangrove, sea grass, coral reef, and beach, and develop local mariculture of oysters, seaweeds, lobsters, crabs, and other marine species.
- Uggupseni, Guna Yala, Panama
Uggupseni (Playon Chico) is an Indigenous village in Guna Yala, the autonomous territory of the Guna people, who live on offshore islands and were never conquered by Europeans. Because of their refusal to be controlled by outsiders or even accept outside investment, they get almost no funding from the Panamanian Government. The Guna are free divers, whose economy is based on lobster sales to outsiders by intermediaries, of which the Guna get only a very small share. Uggupseni has thousands of people crammed into a few hundred meters, with a population density like a huge city.
Lack of land for homes have resulted in traditional widespread mining of living coral reefs for landfill, which was not a problem when there were many corals and few people, but now it is the opposite (Figure 35). About a third of the 50 inhabited islands are now in the process of being abandoned because the Guna can no longer protect their houses from wave destruction, rising sea level, and increased storm strength. But they can protect their islands and unique culture by growing back their coral reefs and Halimeda sand with solar panels. We started small solar-powered Biorock shore protection and lobster habitat projects on a tiny island in Uggupseni in 1996 (Goreau, 2022), and these are still working nearly 30 years later, but we have never been able to find funds for more solar panels and steel to expand the project to protect the Uggupseni population and to restore their lost lobster habitats and populations.
We propose to continue and greatly expand projects so that the Guna can use Biorock as a tool to protect their unique islands, fisheries, and culture, as they wish to do.
 Figure 35. A Guna village washing into the sea, protected from erosion by mining living corals from the reef in front. Photograph 2021 by T. Goreau.
Figure 35. A Guna village washing into the sea, protected from erosion by mining living corals from the reef in front. Photograph 2021 by T. Goreau.
- Barbuda
Barbuda lies in the extreme northeast Caribbean, exposed to the full swell of the North Atlantic with a large shallow bank behind it, with the richest fisheries for lobster and conch in the Caribbean due to the exceptional nursery grounds of Codrington Lagoon, the largest and last pristine lagoon in the Eastern Caribbean, a globally significant migratory bird site, and home to the largest Frigate Bird nesting colony in the Americas. Barbuda is very flat, most barely above sea level, and too dry for groundwater or agriculture, so Barbudans have always lived from fishing. They have managed the most productive fisheries in the region, and feed surrounding islands. As long as they protect their critical nursery grounds in Codrington Lagoon, they can continue to do so.
Hurricane Irma (2017) destroyed all the houses on Barbuda, forcing evacuation of the entire population, and ripped open the barrier beach, made mostly of Halimeda sand, that protected the rich Codrington Lagoon nursery grounds. Previous hurricanes had opened the barrier, but it had always closed back naturally. The Irma breach is getting wider, now about 1.5 kilometers wide, and threatens the Eastern Caribbean’s most pristine and productive fisheries, and the survival of the Barbuda people (Figures 36-37).
The author has worked on coral reef ecology in Barbuda for 30 years, and is working with the Barbuda Council and Barbuda Fisher’s Cooperative to develop urgent projects to grow back the Codrington Lagoon Barrier Beach with Halimeda sand using solar powered Biorock reefs. The future of Barbuda depends on their saving Codrington Lagoon, and getting it designated as a UNESCO World Heritage Site for long term sustainable management and to prevent it being destroyed by rapacious foreign developers, who want to dredge the lagoon for mega yacht marinas, make luxury resort golf courses full of fertilizers, and build luxury resorts on the eroding beach, dumping sewage that will kill the coral reefs and seagrasses in Codrington Lagoon, accelerate the already severe erosion of the island’s beaches, and destroy the very last large pristine lagoon and fisheries in the region (Goreau, 2020).
 Figure 36. The 1.5 mile gap in the beach protecting Codrington Lagoon (lower right) from the open Atlantic (upper left). The red arrow points to a failed resort development the end of the sand bar next to the breach. The blue arrow shows how waves are opening the breach and pushing sand into Codrington Lagoon. Photograph 2020 by T. Goreau.
Figure 36. The 1.5 mile gap in the beach protecting Codrington Lagoon (lower right) from the open Atlantic (upper left). The red arrow points to a failed resort development the end of the sand bar next to the breach. The blue arrow shows how waves are opening the breach and pushing sand into Codrington Lagoon. Photograph 2020 by T. Goreau.
 Figure 37. The failed hotel development on the Codrington Lagoon Barrier Beach collapsing into the gap. Reflection of waves off vertical sea walls may have caused the erosion of the sand bar down current from it. Photograph 2020 by T. Goreau.
Figure 37. The failed hotel development on the Codrington Lagoon Barrier Beach collapsing into the gap. Reflection of waves off vertical sea walls may have caused the erosion of the sand bar down current from it. Photograph 2020 by T. Goreau.
- Saint Jean, Saint Barthelemy
Saint Barthelemy is one of the nearest islands to Barbuda, but instead of being low and flat is very mountainous and rugged, although also too dry for agriculture, so the people survived from fisheries until the recent development of luxury tourism. Pollution has destroyed the lagoons that used to be the fisheries nurseries, and the dead reefs are covered with slimy weedy algae wherever sewage enters the sea. The largest beaches, on bays on the north side, were protected by magnificent coral reefs, and like Barbuda these have been largely killed by global warming as well as by pollution. The eroding dead reef, covered with weeds, can no longer protect the mostly Halimeda beach sand from washing away, and expensive dredging and dumping of sand on beaches by luxury hotels is soon washed away.
Hurricane Irma hit Saint Barthelemy full force right after Barbuda, and devastated all the hotels and most of the homes. The author had been growing a Biorock reef with local environmental groups in I meter depth right on top of the reef crest where the hurricane waves broke that destroyed all hotels and houses on the beach behind it. Astonishingly, the Biorock reef and the corals we were growing at extraordinary rates were completely unharmed (Murcia et al., 2023)! We propose to work with the Saint Barthelemy people to regrow their reef, their Halimeda, their sand, and their largest beach, St. Jean.
- Playa Coral, Matanzas, Cuba
Playa Coral and the Laguna de Maya Marine Sanctuary are on the north shore of Cuba, with narrow fringing coral reefs bathed by the Gulf Stream that drop rapidly into deep waters. These reefs are now the healthiest in the Caribbean, the last place left with diverse corals that survived the record hot bleaching years of 2023 (Goreau & Hayes, 2024) and 2024 (Goreau, Hayes, & Sarkisian, in press).
Unfortunately, despite its name, there is no real beach at “Coral Beach”! Divers climb down a ladder over jagged limestone rocks onto hard eroded fossil reef limestone bottom. Because of the very narrow reef, and steep drop to deep waters offshore, the site is extremely exposed to hurricane waves and severe waves from fronts of cold air from North America, “Northers” or “Arctic Blasts”. The Playa Coral reef is severely sediment starved, waves wash Halimeda sand production rapidly out to deep water through canyons in the living reef, and the little that washes ashore can only accumulate temporarily in crevices and pockets on the hard limestone shore until the next big storm.
Solar powered Biorock Coral Arks in the Marine Protected Area would power the project, aimed to maintain the critical coral biodiversity of the last healthy reef in the Caribbean (https://www.nyas.org/ideas-insights/blog/a-new-perspective-to-sustainable-reef-restoration/), and generate more Halimeda sand so there can become a little more of a white sand beach at Playa Coral (Coral Beach)!
- Seven Mile Beach, Grand Cayman
The Seven Mile Beach on the leeward side of Grand Cayman is the island’s critical tourism and economic treasure. The vast majority of the sand (almost entirely Halimeda) has vanished in recent years, hundreds of meters loss in width, and has vanished entirely where desperate hotels build sea walls that reflect waves and increase erosion (Figure 38).
Grand Cayman is very low and flat, like Barbuda it has no rivers and was surrounded with spectacular reefs. The author has advised Cayman Government environmental officials on coral reef, climate change, and water quality issues for decades. Almost all Cayman corals were killed by high temperatures, and because of crumbling bioerosion of the dead reef coral skeletons, wave erosion has greatly increased, threatening luxury shore front properties with collapse into the sea. Halimeda is abundant on the dead reef and seagrass in front, so the famous former Seven Mile Beach would be an extremely easy site to grow back beaches with Biorock (https://www.globalcoral.org/losing-ground-cayman-facing-race-against-time-to-protect-and-restore-our-beaches/), if funding could be found.

Screenshot
Figure 38. Author filming beach erosion caused by waves reflecting from badly designed seawalls. Seven Mile Beach, Grand Cayman, photograph 2025 by Louise Jenkinson.
-
Discovery Bay, Jamaica
Discovery Bay is named for Columbus’ first landing on Jamaica, but he himself bitterly called it Puerto Seco (Dry Harbour) because his desperately thirsty crew could find no water, since they did not realize the bay was fed with underwater springs (Goreau, 1992). Columbus was terrified of the coral reefs that grew right across the bay, right up to the water level. His boats scraped the coral going in and out and they prayed desperately that they would not sink coming and going.
The author learned to swim as a baby at the Discovery Bay Fishermens’ beach, and has noticed dramatic loss of the beach over 75 years (Figures 39-40). Old photographs show around two thirds of the Halimeda beach sand at Puerto Seco has disappeared, largely after corals died from high temperatures and sewage pollution, and after seawalls and groynes altered shore currents. Establishment of a Discovery Bay sewage plant has greatly improved water quality, eliminating weedy algae, so the incredible growth of Halimeda that used to be there before pollution can now resume, greatly stimulated by Biorock. The beach was recently threatened again by pollution from captive dolphins, but Covid stopped it (Goreau, 2020).
We propose to regrow the sand for Discovery Bay Public Beach, and protect it with Biorock reefs managed as protected fisheries sanctuaries.
 Figure 39. Discovery Bay Fishing Beach, around 1980s, painting by the late Jamaican artist Herbie Rose, photographed by T. Goreau and reproduced with kind permission by the painter. Most of this beach has disappeared.
Figure 39. Discovery Bay Fishing Beach, around 1980s, painting by the late Jamaican artist Herbie Rose, photographed by T. Goreau and reproduced with kind permission by the painter. Most of this beach has disappeared.
 Figure 40. Discovery Bay Fisherman’s Beach 2019, with author talking to local residents. Photograph by Iain Robinson. The captive dolphin pens that made the public beach unusable due to pollution are in the background above the head of the lady at right.
Figure 40. Discovery Bay Fisherman’s Beach 2019, with author talking to local residents. Photograph by Iain Robinson. The captive dolphin pens that made the public beach unusable due to pollution are in the background above the head of the lady at right.
CONCLUSIONS: GROWING SUSTAINABLE SAND AND BEACHES
Until now, beach sand has been a zero-sum game: scarcer and costlier as the best supplies are exhausted, it often was stolen from neighbours. Now, for the first time, Biorock lets us grow back and protect sand to keep pace with sea level rise wherever it is most needed for climate change adaptation without damaging other areas to remove it!
Increased growth of deep reef species like Halimeda goreaui and Halimeda copiosa produces sediments that fall down abyssal slopes, neutralizing ocean acidification when they fall below the lysocline, the depth at which limestone dissolution rapidly increases due to CO2 released from organic matter decomposition on the sea floor. Indeed, floating solar-powered Biorock sand producing reefs could also be grown directly over abyssal waters to greatly enhance plankton and fisheries, while creating new Blue Carbon sinks.
Without urgent changes by policy makers and funding agencies to support effective action to regenerate coastal ecosystems, all coasts and coastal ecosystems will be lost in the near future, starting with coral reefs, and coastal fisheries, tourism, and hopes of a Blue Economy will largely collapse. If funding were in place, beach erosion could be reversed in the tropics wherever Halimeda opuntia can be grown in front of beaches, and in cold waters wherever Maerl sand can be grown. The costs are highly site specific, depending on local wave forces, bottom type, depth, distance, height, etc. At the highest end, with very tall reefs very far from shore, the costs of steel and electrical cable could result in up to around $5,000 per metre of shoreline, less than conventional sea walls that provide none of Biorock’s ecological benefits for sand producing algae, corals, seagrasses, fisheries, biodiversity, and tourism, while lower structures closer to shore will cost a fraction of that amount.
Despite accelerating coastal erosion, there currently is no global funding available for serious large-scale beach and coastal ecosystem regeneration (or even for pilot projects). While people in rich countries are able to move further from the shore following climate disasters, those in poor countries are increasingly forced to live closer to the shore because they have no place to go (Xu et al., 2025). The urgency felt by shore dwellers appears invisible to policymakers and funding agencies that are delusional about the scale and duration of future global climate change impacts, and only visit beaches when it is calm. Their strategy of throwing rocks and concrete in the water after sea walls, roads, building, and airport collapse into the sea will prove ruinously more expensive than precautionary prophylactic protection with Biorock technology to climate proof coastlines, especially in tropical islands.
The small island states have been abandoned by the rich world drowning them. Erosion never sleeps, but politicians won’t wake up and see the light until global sea level rise and increasing storm waves wash away the sand they bury their heads in, fooling themselves that climate change is not real. Their only hope is to grow beaches faster than sea level rise. Biorock sand factories give them that option for the first time.
Cost-effective, life-enhancing Biorock technology is available now to protect tropical beaches and shorelines by solar farming of Halimeda opuntia, but is not being used due to ignorance, stupidity, or greed of developers, policy makers, and funders, who waste money on costly methods that can’t work, grow, or adapt to climate change.
 Figure 41. Many politicians can’t see the light!
Figure 41. Many politicians can’t see the light!
ACKNOWLEDGMENTS
The author gratefully thanks the founders of Halimeda ecology, W. Randolph Taylor, Thomas F. Goreau, and Llewellya Hillis-Colinvaux, for teaching him algae as a child, Wolf Hilbertz for pioneering the electric reefs that now make Biorock beach sand factories possible, and Dr. Viviana Peña Freire (Universidade da Coruña) for identifying maerl algae. Thanks also to Fritz Goreau, Dr. Thomas F. Goreau, Dr. Peter Goreau, Dr. Chowdula Satyanarayana (Zoological Survey of India), Agne Griciute (Velaa Private Island, Maldives), Iain Robinson (Toronto), Louise Jenkinson (Grand Cayman), and Thomas P. Sarkisian for kindly providing photographs and figures. Figures 16-19 were edited by the author from individual frames of a video taken in 2025. The content of this manuscript has been published in part at Global Coral Reef Alliance web site: https://www.globalcoral.org/electrifying-coralline-algae-to-regenerate-white-sand-beaches-and-eroding-islands-against-climate-change/.
CITATIONS
Barbera, C., Bordehore, C., Borg, J.A., Glémarec, M., Grall, J., Hall‐Spencer, J.M., De La Huz, C.H., Lanfranco, E., Lastra, M., Moore, P.G. and Mora, J., 2003. Conservation and management of northeast Atlantic and Mediterranean maerl beds. Aquatic conservation: marine and freshwater ecosystems, 13:S65-S76.
Böhm, E.L., 1973. Coral Reef Project—Papers in Memory of Dr. Thomas F. Goreau. 7. Studies on the Mineral Content of Calcareous Algae. Bulletin of Marine Science, 23:177-190.
Borowitzka, M.A., 1984. Calcification in aquatic plants. Plant, Cell & Environment, 7:457-466.
Borowitzka, M.A. & Larkum, A.W., 1977. Calcification in the green alga Halimeda. I. An Ultrastructure study of thallus development 1. Journal of Phycology, 13:6-16.
Borowitzka, M.A. & Larkum, A.W.D., 1987. Calcification in algae: mechanisms and the role of metabolism. Critical Reviews in Plant Sciences, 6:1-45.
Boss, S.K. & Liddell, W.D., 1987. Patterns of sediment composition of Jamaican fringing reef facies. Sedimentology, 34:77-87.
Bozzeda, F., Ortega, L., Costa, L.L., Fanini, L., Barboza, C.A., McLachlan, A. and Defeo, O., 2023. Global patterns in sandy beach erosion: unraveling the roles of anthropogenic, climatic and morphodynamic factors. Frontiers in Marine Science, 10:1270490.
Castro-Sanguino, C., Bozec, Y.M. & Mumby, P.J., 2020. Dynamics of carbonate sediment production by Halimeda: implications for reef carbonate budgets. Marine Ecology Progress Series, 639:91-106.
Cervino JM, Littler MM, Littler DS, Polson S, Goreau TJ, Brooks B, Smith GW (2005) Identification of microbes associated with coralline lethal algal disease and its relationship to glacial ice melt (global warming). Phytopathology 95(6):120–121 (S120 Publication no P-2005-0005-SSA)
Chenniappan, S., Durairaj, G. and Kumaran, A.V., 2021. Antibacterial activity of Jania rubens from Gulf of Mannar, south coast of India. Indian Journal of Natural Products and Resources (IJNPR)[Formerly Natural Product Radiance (NPR)], 12:451-458.
Colin, P.L., L. J. Bell, & S. Patris, 2008, Helen Reef 2008: An overview. Coral Reef Research Foundation, https://coralreefpalau.org/wp-content/uploads/2017/05/Helen-Reef-Technical-Report-2008.pdf
DeGeorges, A., B. Reilly, & T. Goreau, 2010, Land-sourced pollution with an emphasis on domestic sewage: Lessons from the Caribbean and implications for coastal development on Indian Ocean and Pacific coral reefs, Sustainability, 2: 2919-2949
Drew, E.A., 1983. Halimeda biomass, growth rates and sediment generation on reefs in the central Great Barrier Reef province. Coral Reefs, 2:101-110.
Drew, E.A. & Abel, K.M., 1988. Studies on Halimeda: I. The distribution and species composition of Halimeda meadows throughout the Great Barrier Reef province. Coral Reefs, 6:195-205.
Elhacham, E., Ben-Uri, L., Grozovski, J., Bar-On, YM, & Milo R, Global human-made mass exceeds all living biomass. Nature 588:442–444 (2020). https://doi.org/10.1038/s41586-020-3010-5
Ellis, J. & Solander, D.C., 1786. The natural history of many curious and uncommon zoophytes: collected from various parts of the globe. Benjamin White and Son and Peter Elmsly.
Freile, D., Milliman, J.D. & Hillis, L., 1995. Leeward bank margin Halimeda meadows and draperies and their sedimentary importance on the western Great Bahama Bank slope. Coral Reefs, 14:27-33.
Goreau, T.F., 1959. The ecology of Jamaican coral reefs I. Species composition and zonation. Ecology, 40:67-90.
Goreau, T.F., 1963. Calcium carbonate deposition by coralline algae and corals in relation to their roles as reef‐builders. Annals of the New York Academy of Sciences, 109:127-167.
Goreau, T.F. & Goreau, N.I., 1973. Coral reef project—papers in memory of Dr. Thomas F. Goreau. 17. The ecology of Jamaican coral reefs. II. Geomorphology, zonation, and sedimentary phases. Bulletin of Marine Science, 23:399-464.
Goreau, T.F., Goreau, N.I. & Goreau, T.J., 1979. Corals and coral reefs. Scientific American, 241:124-137.
Goreau, T.F. & Graham, E.A., 1967. A new species of Halimeda from Jamaica. Bulletin of Marine Science, 17:432-441.
Goreau, T.J., 1992. Bleaching and reef community change in Jamaica: 1951–1991. American Zoologist, 32:683-695.
Goreau, T. 2003, Waste Nutrients: Impacts on coastal coral reefs and fisheries, and abatement via land recycling, 28p., UNITED NATIONS EXPERT MEETING ON WASTE MANAGEMENT IN SMALL ISLAND DEVELOPING STATES, Havana, Cuba
Goreau, T.J., 2012, Marine electrolysis for building materials and environmental restoration, p. 273-290 in Electrolysis, J. Kleperis & V. Linkov (Eds.), InTech Publishing, Rijeka, Croatia
Goreau, T.J.F., 2020, Covid halts dolphin pollution in Discovery Bay, Jamaica, https://www.globalcoral.org/covid-ends-dolphin-pollution-in-discovery-bay-jamaica/
Goreau, T.J.F., 2020, Palmetto Point development: potential impacts on Barbuda’s fisheries, https://www.globalcoral.org/palmetto-point-development-potential-impacts-on-barbudas-fisheries/
Goreau-Arango, T.J.F., 2022, Biorock: A technology specifically developed and invented to solve the sea level rise problem forcing Guna people to abandon their islands (in Spanish), https://www.globalcoral.org/biorock-a-technology-specifically-invented-and-developed-to-solve-the-sea-level-rise-problem-forcing-guna-people-to-abandon-their-islands-in-spanish-only/
Goreau, T. J. F., 2022, Coral Reef Electrotherapy: Field Observations, in Frontiers in Marine Science, Cellular Stress Response and Physiological Adaptations of Corals Subjected to Environmental Stressors and Pollutants, D. Seveso, C. Downs, R. Bhagooli (Editors), https://www.frontiersin.org/articles/10.3389/fmars.2022.805113/full
https://www.globalcoral.org/coral-reef-electrotherapy-field-observations/
Goreau, T. J. F., 2023, Biorock electric reefs greatly enhance coral reef structure and function for climate change adaptation, in Corals – Habitat Formers From the Shallow to the Deep, Dr. Giovanni Chimienti, Editor, (http://dx.doi.org/10.5772/intechopen.98140),
https://www.globalcoral.org/electric-reefs-enhance-coral-climate-change-adaptation/
Goreau, T. J. F., 2024, Maldives coral reefs; Past, present, and future: In honour of Maizan Hassan Maniku, https://www.globalcoral.org/maldives-coral-reefs-past-present-and-a-sustainable-blue-future-in-honour-of-maizan-hassan-maniku/
Goreau, T. J. F., 2024, Indigenous/Endogenous Sea Peoples: Climate Change and Environmental Adaptation Prospects, Traditional Knowledge and Climate change – An Environmental Impact on Landscape and Communities, 2023, Ana Penteado, Shambhu Prasad Chakrabarty, & Owais H. Shaikh, (Editors), Springer Nature.
Goreau, T.J. & Hilbertz, W., 2012. Reef restoration using seawater electrolysis in Jamaica. Innovative Methods of Marine Ecosystem Restoration, CRC Press, Boca Raton, pp.35-45.
Goreau, T.J.., & R. K. Trench (Editors), 2012, Innovative Technologies for Marine Ecosystem Restoration, CRC Press
Goreau, T.J.F., & R. L. Hayes, 2021, Global warming triggers coral reef bleaching tipping point, Ambio, https:doi.org/10.1007/s13280-021-01512-2
Goreau, T. J. F. & R. L. Hayes, 2024, 2023 record marine heat waves: Coral Bleaching HotSpot maps reveal global sea surface temperature extremes, coral mortality, and ocean circulation change, Oxford Open Climate Change,
https://academic.oup.com/oocc/article/4/1/kgae005/7666987
Goreau, T. J. F. & P. Prong, 2017, Biorock reefs grow back severely eroded beaches in months, p. 243-263 in T. Wahl, J. E. O. Nilsen, I. Haigh, & S. Brown (Eds.), Coastal Sea Levels, Impacts, and Adaptation, J. Mar. Sci. Eng., 5(4), 48; doi:10.3390/jmse5040048
Goreau T.J, Cervino J, Goreau M, Hayes R, Hayes M, Richardson L, Smith G, DeMeyer K, Nagelkerken I, Garzón-Ferreira J, Gill D, Garrison G, Williams EH, Bunkley-Williams L, Quirolo C, Patterson K, Porter J, Porter K (1998) Rapid spread of disease in Caribbean coral reefs. Rev Biol Trop 46:157–171
Goreau, T. J., E. Hagberg, D. Trevor, & M. Trevor, 2007, Biorock® coral reef restoration and shore protection projects in Majuro, Republic of the Marshall Islands: Preliminary report, https://www.globalcoral.org/biorock-coral-reef-restoration-and-shore-protection-projects-in-majuro-republic-of-the-marshall-islands-preliminary-report/.
Goreau, T.J., R. Lee, L. Nimoho, & I Garae, 2016, Vanuatu Biorock coral reef restoration workshop Havannah Harbour, Efate, June 9-18, 2016 https://www.globalcoral.org/vanuatu-biorock-workshop-june-9-18-2016/
Goreau, T. J. F., Raymond L. Hayes, and Thomas P. Sarkisian, 2025 in press, 2024 Record High Sea Surface Temperatures: Impacts on coral reefs and ocean circulation, Oxford Open Climate Change, preprint at:
https://www.globalcoral.org/2024-record-temperature-effects-on-coral-bleaching-ocean-circulation/
Granier, B., 2012. The contribution of calcareous green algae to the production of limestones: a review. Geodiversitas, 34:35-60.
Hilbertz, W.H., 1992. Solar-generated building material from seawater as a sink for carbon. Ambio, 21:126-129
Hillis-Colinvaux, L., 1980. Ecology and taxonomy of Halimeda: primary producer of coral reefs. Advances in Marine Biology, 17:1-327. Academic Press.
Hillis, L.W., Engman, J.A. & Kooistra, W.H., 1998. Morphological and molecular phylogenies of Halimeda (Chlorophyta, Bryopsidales) identify three evolutionary lineages. Journal of Phycology, 34:669-681.
Hunter, C.L. and Evans, C.W., 1995. Coral reefs in Kaneohe Bay, Hawaii: two centuries of western influence and two decades of data. Bulletin of Marine Science, 57:501-515.
Jensen, P.R., Gibson, R.A., Littler, M.M. & Littler, D.S., 1985. Photosynthesis and calcification in four deep-water Halimeda species (Chlorophyceae, Caulerpales). Deep Sea Research Part A. Oceanographic Research Papers, 32:451-464.
Johns, H.D. & Moore, C.H., 1988. Reef to basin sediment transport using Halimeda as a sediment tracer, Grand Cayman Island, West Indies. Coral Reefs, 6:187-193.
Jormelu, K., E. Hagberg, & T.J. Goreau, 2010, Shore protection in the Republic of the Marshall Islands – Pilot Project report. https://www.globalcoral.org/shore-protection-in-the-republic-of-the-marshall-islands-pilot-project-report/
Kooistra, W.H., Coppejans, E.G. & Payri, C., 2002. Molecular systematics, historical ecology, and phylogeography of Halimeda (Bryopsidales). Molecular phylogenetics and evolution, 24:121-138.
Kooistra, W.H. & Verbruggen, H., 2005. Genetic patterns in the calcified tropical seaweeds Halimeda opuntia, H. distorta, H. hederacea, and H. minima (bryopsidales, chlorophyta) provide insights in species boundaries and interoceanic dispersal 1. Journal of phycology, 41:177-187.
Naiel, M.A., Zaki, E.M., Khames, D.K., Ismael, N.E., Meng, X., Elian, S.M. & Arisha, A.H., 2025. Effects of Dietary Supplementation with Halimeda opuntia Powder on Growth Performance, Digestibility, Immune Response, Antioxidant Defensive System and Gene Regulation of Oreochromis niloticus. Journal of Applied Phycology, 1-15.
Land, L.S., 1979. The fate of reef-derived sediment on the north Jamaican island slope. Marine Geology, 29:55-71.
Liddell, W.D. & Ohlhorst, S.L., 1981. Geomorphology and community composition of two adjacent reef areas, Discovery Bay, Jamaica.
Liddell, W.D. & Ohlhorst, S.L., 1987. Patterns of reef community structure, North Jamaica. Bulletin of Marine Science, 40:311-329.
Liddell, W.D. & Ohlhorst, S.L., 1988. Hard substrata community patterns, 1-120 M, North Jamaica. Palaios:413-423.
Liddell, W.D. & Ohlhorst, S.L., 1992. Ten years of disturbance and change on a Jamaican fringing reef. Proc 7th Int Coral Reef Symp, Guam, 1:144-150).
LIFE 1946, What Science learned at Bikini
Littler MM, Littler DS,1995, Impact of CLOD pathogen on Pacific coral reefs. Science 267:1356–1360
Littler, D.S. & M. M. Littler, 2000, Caribbean Reef Plants, OffShore Graphics Inc., Washington DC
Littler, D.S. & M. M. Littler, 2003, South Pacific Reef Plants, OffShore Graphics Inc., Washington DC
Luijendijk, A., Hagenaars, G., Ranasinghe, R., Baart, F., Donchyts, G. and Aarninkhof, S., 2018. The state of the world’s beaches. Scientific reports, 8:6641.
Mackenzie, F.T., & A. Lerman, 2006, Carbon in the Geobiosphere: Earth’s Outer Shell, Springer, Dordrecht, the Netherlands.
McCoy, S.J. and Kamenos, N.A., 2015. Coralline algae (Rhodophyta) in a changing world: integrating ecological, physiological, and geochemical responses to global change. Journal of Phycology, 51:6-24.
Milliman, J.D., 1974, Marine Carbonates, Springer-Verlag, Berlin.
Milliman, J.D., 1993. Production and accumulation of calcium carbonate in the ocean: Budget of a nonsteady state. Global Biogeochemical Cycles, 7:927-957.
Milliman, J.D. & Droxler, A.W., 1995. Calcium carbonate sedimentation in the global ocean: linkages between the neritic and pelagic environments. Oceanography, 8:92-94.
Moore, C.H., Graham, E.A. & Land, L.S., 1976. Sediment transport and dispersal across the deep fore-reef and island slope (–55 m to–305 m), Discovery Bay, Jamaica. Journal of Sedimentary Research, 46:174-187.
Muñoz, R.C. and Motta, P.J., 2000. Interspecific aggression between two parrotfishes (Sparisoma, Scaridae) in the Florida Keys. Copeia, 2000:674-683.
Murcia-Eslava, J.D., Y. Nuñez-Sepulveda, T. Farago Szekeres, & T. Goreau-Arango, 2023, Field Report: Visit to coral reef projects with Biorock mineral accretion technology, Saint Barthelemy, Caribbean, https://www.globalcoral.org/biorock-grows-back-saint-barthelemy-reefs-2023-cres-report/
Neumann, A.C. & Land, L.S., 1975. Lime mud deposition and calcareous algae in the Bight of Abaco, Bahamas; a budget. Journal of Sedimentary Research, 45:763-786.
Niedenthal, J., 2013, For the Good of Mankind: A History of the People of Bikini and Their Islands,
Peña, V., Harvey, B.P., Agostini, S., Porzio, L., Milazzo, M., Horta, P., Le Gall, L. & Hall‐Spencer, J.M., 2021. Major loss of coralline algal diversity in response to ocean acidification. Global Change Biology, 27:4785-4798.
Peña, V, & Barbara, I., 2008, Maerl community in the north-western Iberian peninsula: A review of floristic studies and long term changes, Aquatic Conservation: Marine and Freshwater Ecosystems, 18:339-366
Pongparadon, S., Zuccarello, G.C., Phang, S.M., Kawai, H., Hanyuda, T. & Prathep, A., 2015. Diversity of Halimeda (Chlorophyta) from the Thai–Malay Peninsula. Phycologia, 54:349-366.
Rangel-Buitrago, N., Neal, W., Pilkey, O. and Longo, N., 2023. The global impact of sand mining on beaches and dunes. Ocean & Coastal Management, 235:106492.
Reolid, J., Bialik, O.M., Lindhorst, S., Eisermann, J.O., Petrovic, A., Hincke, C., Beaman, R.J., Webster, J.M. & Betzler, C., 2024. A new type of Halimeda bioherm on the Queensland Plateau, NE Australia. Coral Reefs, 43:801-821.
Roberts, H.H., Phipps, C.V. & Effendi, L., 1987. Halimeda bioherms of the eastern Java Sea, Indonesia. Geology, 15:371-374.
Schubert, N., Alvarez-Filip, L. & Hofmann, L.C., 2023. Systematic review and meta-analysis of ocean acidification effects in Halimeda: Implications for algal carbonate production. Climate Change Ecology, 4:100059.
Schoemaker, K., 2025. Sand‐Hungry: Accumulations, Erosions, and the Self‐Feeding Logic of Beach Renourishment. Antipode, 57:1105-1125.
Sloane, H., 1707, Natural History of Jamaica (a voyage to the islands Madera, S. Christophers, and Jamaica with the natural history of the herbs and trees, four footed beasts, fishes, birds, insects, reptiles), London, 2017 reprint, Facsimile Publisher, Delhi, India.
Taylor, W.R., 1962. Two undescribed species of Halimeda. Bulletin of the Torrey Botanical Club, pp.172-177.
Vásquez-Elizondo, R.M. and Enríquez, S., 2016. Coralline algal physiology is more adversely affected by elevated temperature than reduced pH. Scientific Reports, 6:19030.
Verbruggen, H. & Kooistra, W.H., 2004. Morphological characterization of lineages within the calcified tropical seaweed genus Halimeda (Bryopsidales, Chlorophyta). European Journal of Phycology, 39:213-228.
Verbruggen, H., De Clerck, O., Cocquyt, E., Kooistra, W.H. & Coppejans, E., 2005. Morphometric Taxonomy of Siphonous Green Algae: a Methodological Study Within the Genus Halimeda (Bryopsidales) 1. Journal of Phycology, 41:126-139.
Verbruggen, H., Tyberghein, L., Pauly, K., Vlaeminck, C., Nieuwenhuyze, K.V., Kooistra, W.H., Leliaert, F. & Clerck, O.D., 2009. Macroecology meets macroevolution: evolutionary niche dynamics in the seaweed Halimeda. Global Ecology and Biogeography, 18(4), pp.393-405.
Vogel, N., Fabricius, K.E., Strahl, J., Noonan, S.H.C., Wild, C. & Uthicke, S., 2015. Calcareous green alga Halimeda tolerates ocean acidification conditions at tropical carbon dioxide seeps. Limnology and Oceanography, 60:263-275.
Vousdoukas, M.I., Ranasinghe, R., Mentaschi, L., Plomaritis, T.A., Athanasiou, P., Luijendijk, A. & Feyen, L., 2020. Sandy coastlines under threat of erosion. Nature Climate Change, 10:260-263.
Wanless, H.R. and Maier, K.L., 2007. An evaluation of beach renourishment sands adjacent to reefal settings, southeast Florida. Southeastern Geology, 45:25-42.
Xu, L., Yang, X., Chen, D., Sornette, D., Prishchepov, A.V., Ding, S., Pang, W., Suryanto Pribadi, K., Di, B. and Wang, X., 2025. Global coastal human settlement retreat driven by vulnerability to coastal climate hazards. Nature Climate Change:1-11.
Zhang, H., Wang, X., Qu, M., Yu, H., Yin, J., Liu, X., Liu, Y., Zhang, B., Zhang, Y., Wei, Z. & Yang, F., 2024. Genome of Halimeda opuntia reveals differentiation of subgenomes and molecular bases of multinucleation and calcification in algae. Proceedings of the National Academy of Sciences, 121(39), p.e2403222121.